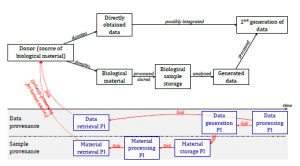
The exchange of data and samples in clinical practice and biomedical research is increasingly common and necessary, but requires an effective evaluation of data and samples quality, which affects the reliability and reproducibility of the results obtained from their analysis. In this context CRS4 collaborates with BBMRI-ERIC (the European Biobank Infrastructure) to define an ISO standard that models the provenance of data and samples, defining the fundamental information to describe in detail the processes of generation, treatment and analysis in the biotechnology domain (ISO/WD TS 23494 Biotechnology - Provenance information model for biological material and data).
In May 2020, the first part of the standard (Design concepts and general requirements) has been approved by the relevant Technical Committee (ISO/TC 276 Biotechnology) and proceeds in the evaluation process, while, in parallel, the work on the other parts of the standard is progressing.